
产品介绍
FluorPen手持式叶绿素荧光仪用于实验室和野外快速测量植物叶绿素荧光参数,具有便携性强、精确度高、性价比高等特点;双键操作,具显示屏,可以贮存1000次测量数据,广泛应用于研究植物的光合作用、胁迫监测、杀虫剂实验或变异筛选,还可用于生物检测,如通过不同植物对土壤或水质污染的叶绿素荧光响应,找出敏感植物作为生物传感器用于生物检测。其分离叶夹版配备多个暗适应叶夹,便于在白天光照下进行各种荧光测量。
应用领域
适用于光合作用研究和教学,植物及分子生物学研究,农业、林业,生物技术领域等。研究内容涉及光合活性、胁迫响应、农药药效测试、突变等。
· 植物光合特性和代谢紊乱筛选
· 生物和非生物胁迫的检测
· 植物抗胁迫能力或者易感性研究
· 代谢混乱研究
· 长势与产量评估
· 植物——微生物交互作用研究
· 植物——原生动物交互作用研究
功能特点
§ 结构紧凑、便携性强,LED光源、检测器、控制单元集成于仅手机大小的仪器内,重量仅180g
§ 功能强大,是叶绿素荧光技术的高端结晶产品,具备了大型荧光仪的所有功能,可以测量所有叶绿素荧光参数
§ 内置了所有通用叶绿素荧光分析实验程序,包括两套荧光淬灭分析程序、3套光响应曲线程序、OJIP–test等
§ 高时间分辨率,可达10万次每秒,自动绘出OJIP曲线并给出26个OJIP–test参数
§ 可配备GPS模块,输出待时间戳和地理位置的叶绿素荧光参数图表
§ FluorPen专业软件功能强大,可下载、展示叶绿素荧光参数图表,还可实现遥控功能
§ 配置灵活,可选配蓝牙通讯或USB通讯,标配叶夹为固定式,还可选配分离叶夹式(适合于白天多个叶夹暗适应)、透明叶夹式(无需暗适应的情况下)等
§ 可选配野外自动监测式FluorPen,防水防尘设计
工作原理
利用调制式荧光测量技术,采用LED光源,选择仪器内置的给光方案测量并计算叶绿素荧光的各种参数。
实验过程和测量参数
· Ft:瞬时叶绿素荧光、暗适应完成后Ft=Fo
· QY:光量子效率,表示光系统II 的效率,等于Fv/Fm(暗适应完成的样品)或Fv’/Fm’ (光适应完成的样品)
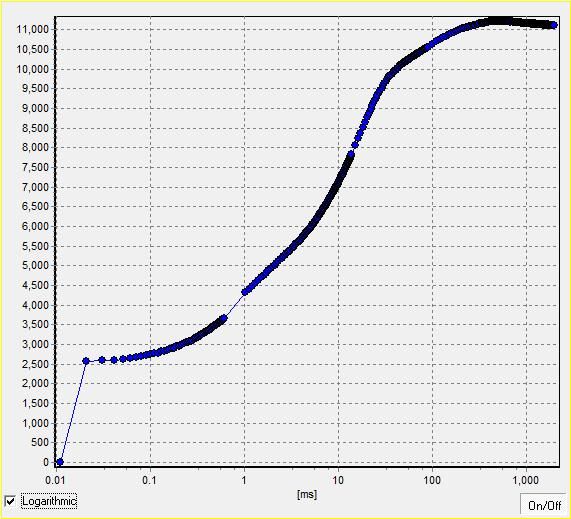
· OJIP:叶绿素荧光瞬时OJIP曲线是反应光合作用过程中植物生理时间过程的重要信号。
· NPQ:非光化学淬灭,表示光合作用中叶绿素吸收光能后以热形式散失掉的部分。
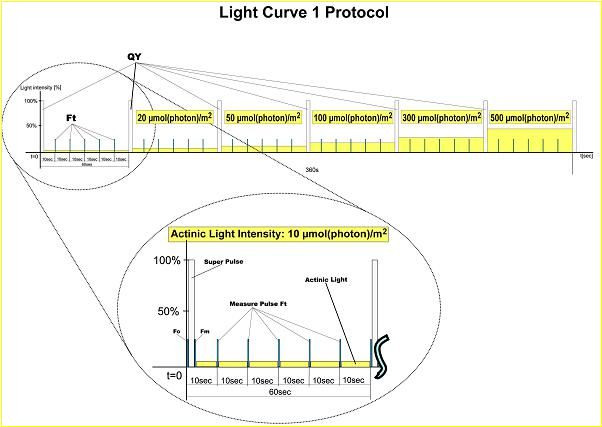
· 光曲线:Qy对不同光强的适应曲线。
· PAR测量:可在荧光仪上显示PAR值,可计算20次检测值的平均。
· 可选配GPS模块
技术参数
· 测量参数包括F0、Ft、Fm、Fm’、QY_Ln、QY_Dn、NPQ、Qp、Rfd、PAR、Area、Mo、Sm、PI、ABS/RC等50多个叶绿素荧光参数,及3种给光程序的光响应曲线、2种荧光淬灭曲线、OJIP曲线等
· OJIP–test时间分辨率为10µs(每秒10万次),给出OJIP曲线和26个参数,包括F0、Fj、Fi、Fm、Fv、Vj、Vi、Fm/F0、Fv/F0、Fv/Fm、Mo、Area、Fix Area、Sm、Ss、N、Phi_Po、Psi_o、Phi_Eo、Phi–Do、Phi_Pav、PI_Abs、ABS/RC、TRo/RC、ETo/RC、DIo/RC等
· 3种给光程序光响应曲线,20多个叶绿素荧光参数
· 2种荧光淬灭曲线,20多个参数
· PAR传感器:读数单位µmol(photons)/m².s,可显示读数,检测范围400-700 nm
· 测量光:光强可调
· 光化学光:0–1000µmol(photons)/m².s可调
· 饱和光:0–3000µmol(photons)/m².s可调
· 光源:标准配置蓝光470nm,可根据需求配备不同波长的LED光源
· 检测器:PIN光电二极管,697–750nm滤波器
· 尺寸大小:超便携,只有手机大小,12cm×5.7cm×3cm,重量仅约180g
· 存贮:容量4Mb,可内存100000数据点
· 显示:2×8字符液晶显示屏,双键操作,待机5分钟自动关闭
· 供电:4节AAA电池可持续使用70小时
· 工作条件:0–55℃,0–95%相对湿度(无凝结水)
· 存贮条件:-10–60℃,0–95%相对湿度(无凝结水)
· 下载方式:可选配蓝牙或USB
· FluorPen软件,用于数据下载、分析和图表显示
· 备选GPS定位模块
操作软件与实验结果
产地:捷克
参考文献
1. Duarte B, et al, 2016. Disentangling the photochemical salinity tolerance in Aster tripolium L.: connecting biophysical traits with changes in fatty acid composition. Plant Biology, DOI: 10.1111/plb.12517
2. Ajigboye OO, et al, 2016. Altered gene expression by sedaxane increases PSII efficiency, photosynthesis and growth and improves tolerance to drought in wheat seedlings. Pesticide Biochemistry and Physiology, http://dx.doi.org/10.1016/j.pestbp.2016.09.008
3. Ajigboye OO, et al, 2016. Chlorophyll fluorescence parameters allow the rapid detection and differentiation of plant responses in three different wheat pathosystems. Functional Plant Biology 43(4):356-369
4. Ruiz‐Lozano JM, et al, 2016. Arbuscular mycorrhizal symbiosis induces strigolactone biosynthesis under drought and improves drought tolerance in lettuce and tomato. Plant, Cell & Environment 39(2):441–452
5. Dyakov MY, Insarova ID, Kharabadze DE, Ptushenko VV, And Shtaer OV, 2015. Influence of extreme ambient temperatures and anaerobic conditions on Peltigera aphthosa (L.) Willd. viability. Life sciences in space research 7: 66-72
6. Ptushenko VV, Avercheva OV, Bassarskaya EM, Berkovich YA, Erokhin AN, Smolyanina SO, And Zhigalova TV, 2015. Possible reasons of a decline in growth of Chinese cabbage under a combined narrowband red and blue light in comparison with illumination by high-pressure sodium lamp. Scientia Horticulturae 194:267-277
7. Orekhov DI, Yakovleva SN, Goryachev FF, Protopopov FF, And Alekseev AA, 2015. The use of parameters of Chlorophyll a fluorescence induction to evaluate the state of plants under anthropogenic load. Biophysics 60(2):263–268
8. Fesenko IA, Arapidi GP, Skripnikov AY, et al, 2015. Specific pools of endogenous peptides are present in gametophore, protonema, and protoplast cells of the moss Physcomitrella patens. Pesticide Biochemistry and Physiology 15:1-16
9. Humplík JF, Lazár D, Fürst T, et al, 2015. Automated integrative high-throughput phenotyping of plant shoots: a case study of the cold-tolerance of pea (Pisum sativumL.). Plant Methods 11:1-11
10. Tripathi DK, Singh VP, Prasad SM. et al, 2015. Silicon-mediated alleviation of Cr(VI) toxicity in wheat seedlings as evidenced by chlorophyll florescence, laser induced breakdown spectroscopy and anatomical changes. Ecotoxicology and Environmental Safety 113:133-144
11. Ajigboye OO, Murchie E, Ray RV, 2014. Foliar application of isopyrazam and epoxiconazole improves photosystem II efficiency, biomass and yield in winter beat. Pesticide Biochemistry and Physiology 114:52–60
12. Calderón R, Lucena C, Trapero-Casas JL, et al, 2014. Soil temperature determines the reaction of olive cultivars to Verticillium dahliae pathotypes. PLoS One 9, DOI:10.1371/journal.pone.0110664
13. Jueterbock A, Kollias S, Smolina I, et al, 2014. Thermal stress resistance of the brown alga Fucus serratusalong the North-Atlantic coast: Acclimatization potential to climate change. Marine Genomics 13:27-36.
14. Ptushenko VV, Ptushenko OS. And Tikhonov AN,2014. Chlorophyll Fluorescence Induction, Chlorophyll Content, and Chromaticity Characteristics of Leaves as Indicators of Photosynthetic Apparatus Senescence in Arboreous Plants. Biochemistry 79(3):260-272
附:OJIP参数及计算公式
Bckg = background
Fo: = F50µs; fluorescence intensity at 50 µs
Fj: = fluorescence intensity at j-step (at 2 ms)
Fi: = fluorescence intensity at i-step (at 60 ms)
Fm: = maximal fluorescence intensity
Fv: = Fm - Fo (maximal variable fluorescence)
Vj = (Fj - Fo) / (Fm - Fo)
Fm / Fo = Fm / Fo
Fv / Fo = Fv / Fo
Fv / Fm = Fv / Fm
Mo = TRo / RC - ETo / RC
Area = area between fluorescence curve and Fm
Sm = area / Fm - Fo (multiple turn-over)
Ss = the smallest Sm turn-over (single turn-over)
N = Sm . Mo . (I / Vj) turn-over number QA
Phi_Po = (I - Fo) / Fm (or Fv / Fm)
Phi_o = I - Vj
Phi_Eo = (I - Fo / Fm) . Phi_o
Phi_Do = 1 - Phi_Po - (Fo / Fm)
Phi_Pav = Phi_Po - (Sm / tFM); tFM = time to reach Fm (in ms)
ABS / RC = Mo . (I / Vj) . (I / Phi_Po)
TRo / RC = Mo . (I / Vj)
ETo / RC = Mo . (I / Vj) . Phi_o)
DIo / RC = (ABS / RC) - (TRo / RC)
|